Symbol
Definition
Relevant Standard
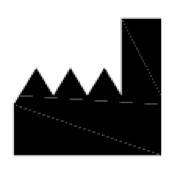
Manufacturer.
Indicates the medical device manufacturer.
ISO 15223-1:2020. 5.1.1
ISO 7000 reg. no. 3082

Authorized representative in the European Community.
Indicates the Authorized representative in the European Community.
ISO 1552-1:2020. 5.1.2
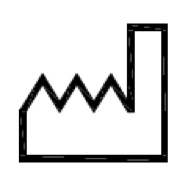
Date of manufacture.
Indicates the date when the medical device was manufactured
ISO 15223-1:2020. 5.1.3
ISO 7000 reg. no. 2497
2004-01-15
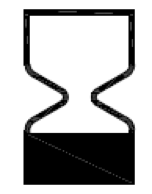
Use-by date.
Indicates the date after which the medical device is not to be used.
ISO 15223-1:2020. 5.1.4
ISO 7000 reg. no. 2607
2004-01-15

Batch code.
Indicates the manufacturer's batch code so that the batch or lot can be identified.
ISO 15223-1:2020. 5.1.5
ISO 7000 reg. no. 2492
2004-01-15

Catalogue number.
Indicates the manufacturer's catalogue number so that the medical device can be identified.
ISO 15223-1:2020. 5.1.6
ISO 7000 reg. no. 2493
2004-01-15

Serial number.
Indicates the manufacturer's serial number so that a specific medical device can be identified.
ISO 15223-1:2020. 5.1.7
ISO 7000 reg. no. 2498
2004-01-15

Importer.
Indicates the entity importing the medical device into the locale
ISO 15223-1:2020. 5.1.8
ISO 7000-3725
2019-11-01
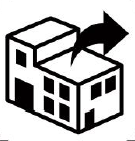
Distributor.
Indicates the entity distributing the medical device into the locale
ISO 15223-1:2020. 5.1.9
ISO 7000-3724
2019-11-01

Country of Manufacture.
To identify the country of manufacture of products
ISO 15223-1:2020. 5.1.11
ISO 7000-6049
2012-07-14

Sterile.
Indicates a medical device that has been subjected to a sterilization process.
ISO 15223-1:2020. 5.2.1
ISO 7000-2499
2004-01-15

Non-sterile.
Indicates a medical device that has not been subjected to a sterilization process.
ISO 15223-1:2020. 5.2.7
ISO 7000 reg. no. 2609
2004-01-15
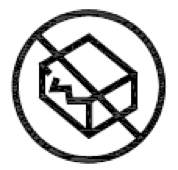
Do not use if package is damaged.
Indicates a medical device that should not be used if the package has been damaged or opened.
ISO 15223-1:2020. 5.2.8
ISO 7000 reg. no. 2606
2004-01-15

Fragile, handle with care.
Indicates a medical device that can be broken or damaged if not handled carefully.
ISO 15223-1:2020. 5.3.1
ISO 7000 reg. no. 0621
2014-06-04
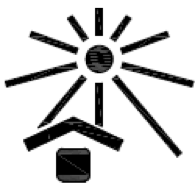
Keep away from sunlight.
Indicates a medical device that needs protection from light sources.
ISO 15223-1:2020. 5.3.2
ISO 7000 reg. no. 0624
2014-06-04

Keep away from sunlight.
Indicates a medical device that needs protection from light sources.
ISO 15223-1:2020. 5.3.2
ISO 7000 reg. no. 0624
2014-06-04
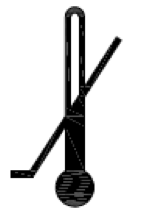
Lower limit of temperature.
Indicates the lower limit of temperature to which the medical device can be safely exposed.
ISO 15223-1:2020. 5.3.5
ISO 7000 reg. no. 0534
2004-01-15
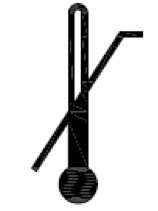
Upper limit of temperature.
Indicates the upper limit of temperature to which the medical device can be safely exposed.
ISO 15223-1:2020. 5.3.6
ISO 7000 reg. no. 0533
2004-01-15
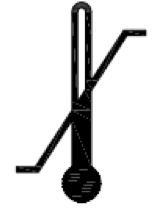
Temperature limit.
Indicates the temperature limits to which the medical device can be safely exposed.
ISO 15223-1:2020. 5.3.7
ISO 7000 reg. no. 0632
2014-06-04

Humidity limitation.
Indicates the range of humidity to which the medical device can be safely exposed.
ISO 15223-1:2020. 5.3.8
ISO 7000 reg. no. 2620
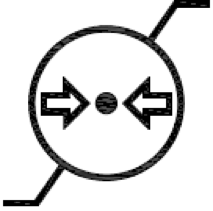
Atmospheric pressure limitation.
Indicates the range of atmospheric pressure to which the medical device can be safely exposed.
ISO 15223-1:2020. 5.3.9
ISO 7000 reg. no. 2621
2004-01-15

Biological risks.
Indicates that there are potential biological risks associated with the medical device.
ISO 15223-1:2020. 5.4.1
ISO 7000 reg. no. 2621
2004-01-15
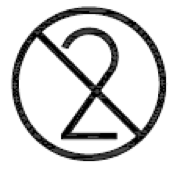
Do not re-use.
Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure
ISO 15223-1:2020. 5.4.2
ISO 7000 reg. no. 2621
2004-01-15
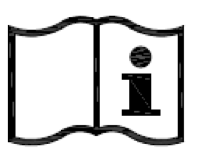
Consult instructions for use.
Indicates the need for the user to consult the instructions for use.
ISO 15223-1:2020. 5.4.3
ISO 7000 reg. no. 2621
2004-01-15
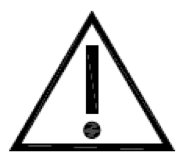
Caution.
Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself.
ISO 15223-1:2020. 5.4.4
ISO 7000 reg. no. 0434A
2004-01-15
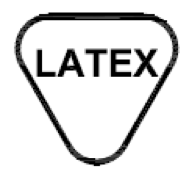
Contains or presence of natural rubber latex.
Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device.
ISO 15223-1:2020. 5.4.5
ISO 7000, symbol 2725
2005-09-08

Contains a medicinal substance.
Indicates a medical device that contains or incorporates a medicinal substance
ISO 15223-1:2020. 5.4.7
ISO 7000-3702
2019-10-18
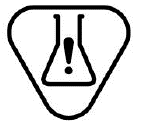
Contains Hazardous substances.
Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties
ISO 15223-1:2020. 5.4.10
ISO 7000-3723
2019-11-01
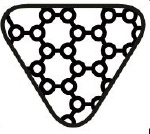
Contains nano materials.
Indicates a medical device that contains nano materials
ISO 15223-1:2020. 5.4.7
ISO 7000-3702
2019-10-18
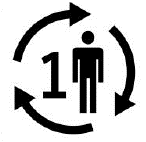
Single patient multiple use.
Indicates a medical device that may be used multiple times (multiple procedures) on a single patient
ISO 15223-1:2020. 5.4.12
ISO 7000-3706
2019-10-18

For IVD performance evaluation only.
Indicates an IVD device that is intended to be used only for evaluating its performance characteristics before it is placed on the market for medical diagnostic use.
ISO 15223-1:2020. 5.5.6
ISO 7000- 3083
2011-10-03

Sampling site.
Indicates a medical device or blood processing application that includes a system dedicated to the collection of samples of a given substance stored in the medical device or blood container.
ISO 15223-1:2020. 5.6.1
ISO 7000 reg. no. 2715
2005-09-08

Fluid path.
Indicates the presence of a fluid path.
ISO 15223-1:2020. 5.6.2
ISO 7000 reg. no. 2722
2005-09-08

Non-pyrogenic.
Indicates a medical device that is non-pyrogenic.
ISO 15223-1:2020. 5.6.3
ISO 7000 reg. no. 2724
2005-09-08
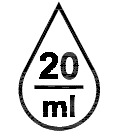
Drops per milliliter.
Indicates the number of drops per millilitre.
ISO 15223-1:2020. 5.6.4
ISO 7000 reg. no. 2726
2005-09-08

Liquid filter with pore size.
Indicates an infusion or transfusion system of the medical device that contains a filter of a particular nominal pore size.
ISO 15223-1:2020. 5.6.5
ISO 7000 reg. no. 2727
2005-09-08
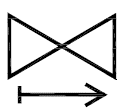
One-way valve
Indicates a medical device with a valve that allows flow in only one direction.
ISO 15223-1:2020. 5.6.6
ISO 7000 reg. no. 2728
2005-09-08
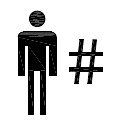
Patient number.
Indicates a unique number associated with an individual patient.
ISO 15223-1:2020. 5.7.1
ISO 7000 reg. no. 2610
2004-01-15

Patient information website.
Indicates a website where a patient can obtain additional information on the medical product
ISO 15223-1:2020. 5.7.4
ISO 7000-3705
2019-10-18

Medical Device.
Indicates the item is a medical device
ISO 15223-1:2020. 5.7.7
Repackaging
To identify that a modification to the original medical device packaging configuration has occurred
ISO 15223-1:2020. 5.7.9
ISO 7000-3727
2019-11-01

Unique Device Identifier.
Indicates a carrier that contains Unique Device Identifier information
ISO 15223-1:2020. 5.7.10

UK Conformity Assessed Marking
Indicates conformity with local laws and regulations in Great Britain (England, Wales and Scotland)
UK MDR 2002

Conformitè Europëenne / European Conformity
Indicates conformity with local laws and regulations within the European Economic Area








 France
France Australia
Australia

